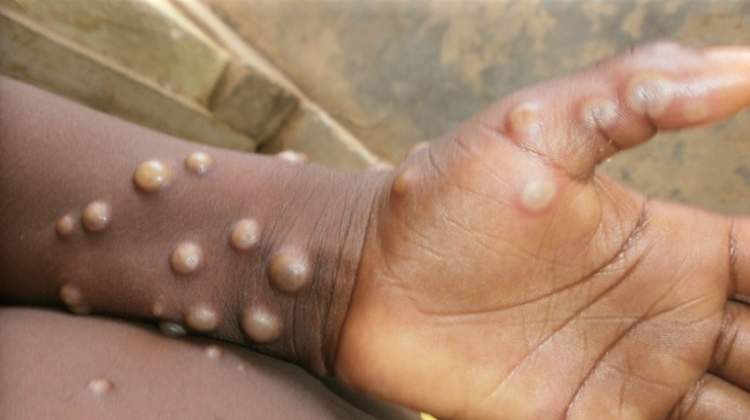
WHO Approves First Mpox Diagnostic Test
The World Health Organisation has taken a crucial step towards improving global access to mpox testing by listing the first mpox in vitro diagnostic test under its Emergency Use Listing procedure. The approved test, Alinity m MPXV assay, is manufactured by Abbott Molecular Inc. and has been designed to detect monkeypox virus DNA from human skin lesion swabs.
Mpox, which has been spreading rapidly in Africa, requires timely treatment, care, and control. However, limited testing capacity and delays in confirming cases have been a significant challenge in the region. According to the WHO, over 30,000 suspected cases have been reported across Africa this year, with the highest numbers in the Democratic Republic of the Congo, Burundi, and Nigeria. In the Democratic Republic of the Congo, only 37% of suspected cases have been tested.
The Alinity m MPXV assay is a real-time PCR test that is intended for use by trained clinical laboratory personnel. By detecting DNA from pustular or vesicular rash samples, health workers can confirm suspected mpox cases efficiently and effectively.
Dr Yukiko Nakatani, WHO Assistant Director-General for Access to Medicines and Health Products, highlighted the significance of this development, stating that increasing access to quality-assured medical products is essential for containing the spread of the virus and protecting underserved regions.
The EUL process is designed to fast-track the availability of life-saving medical products during public health emergencies. It assesses the quality, safety, and performance of essential health products, guiding procurement agencies and WHO Member States in making informed decisions for emergency procurement.
While WHO has received three additional submissions for EUL evaluation, discussions are ongoing with other manufacturers of mpox IVDs to ensure a wider range of quality-assured diagnostic options are available. The EUL for the Alinity m MPXV assay will remain valid as long as the Public Health Emergency of International Concern is in effect.
This approval marks an important milestone in the fight against mpox, ensuring that more countries can access quality-assured diagnostic tests to control the spread of the virus.