A significant milestone has been reached in the fight against Lassa fever, a deadly virus endemic in West Africa, with the first volunteer receiving a dose in a first-in-human trial of Oxford’s Lassa vaccine. The trial, conducted by the Oxford Vaccine Group and funded by the Coalition for Epidemic Preparedness Innovations (CEPI), aims to assess the safety and immune response of the ChAdOx1 Lassa vaccine.
The vaccine, developed by researchers at the Pandemic Sciences Institute, University of Oxford, utilizes the same viral vector platform as the Oxford/AstraZeneca COVID-19 vaccine, which has been estimated to have saved six million lives in its first year. The trial will involve 31 participants aged 18-55 and is being conducted in Oxford, with a second phase 1 trial set to commence in Ghana early next year.
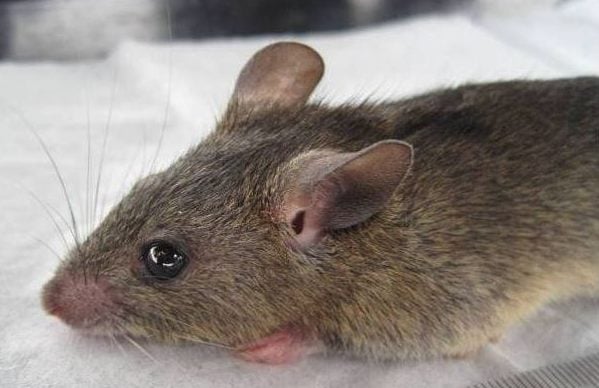
Lassa fever, caused by the Lassa virus, is primarily spread by rodents and can result in severe illness, including deafness, bleeding, and death. The World Health Organisation has identified Lassa fever as a priority pathogen in need of research and development due to its potential to cause large outbreaks. In 2025, Nigeria has recorded 995 confirmed cases and 184 deaths from the disease.
The Oxford Vaccine Group, part of the Department of Paediatrics at the University of Oxford, has a proven track record in vaccine development, having led the rapid clinical development of COVID-19 vaccinations during the pandemic. CEPI, an innovative partnership between public, private, philanthropic, and civil organisations, has supported the early preclinical development of the vaccine and is committed to accelerating the development of vaccines against epidemic and pandemic threats.
The launch of the trial has been welcomed by health experts, with Prof. Maheshi Ramasamy, Chief Investigator of the trial, stating that vaccines are a powerful tool in global health, saving lives and stopping outbreaks. Dr Katrin Ramsauer, Lassa Disease Programme Lead at CEPI, noted that the launch of the clinical study brings us closer to a future where communities no longer live in fear of Lassa fever.
The development of a Lassa fever vaccine is a crucial step towards protecting vulnerable communities from the devastating impact of the disease. With regional leadership and coordination by the Lassa fever Coalition, plans are progressing to advance a Lassa vaccine to licensure, with the goal of equitable introduction across the affected region. The West African Health Organisation, in partnership with CEPI and other stakeholders, is working to accelerate the development and introduction of Lassa fever vaccines, bringing hope to communities affected by the disease for over half a century.